Welcome! You are encouraged to register with the site and login (for free). When you register, you support the site and your question history is saved.
Review: Molecules of Carbon Dioxide in a Sample
Explanation
In this question,
we are working with a ratio that doesn't change, so we can write two instances
of this ratio and set them equal:
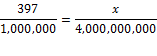
Counting zeroes:
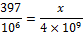
We can cross-multiply and solve:
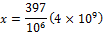
![]()
Hearing "approximately" and seeing the answer choices, we
can take ![]() .
.
![]()
![]()
The correct answer is (C).
If you believe you have found an error in this question or explanation, please contact us and include the question title or URL in your message.